Hippo
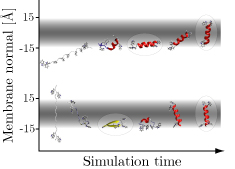
Hippo is a software package for simulation and analysis of bio-molecules at an atomic level. It has been specifically developed for very efficient protein folding studies in aqueous and membrane environments. The code is very fast due to optimized and hand-coded assembly routines which make use of fast multi-media instructions on modern x86 cpus. Hippo is (partially) parallelized (using industry-standard openMP).
Download
Binaries will be publicly available from the Biowerkzeg.com download page for Linux, Windows, and Mac OS X (Intel platform only). For immediate access to beta-versions please email martin-AT-ulmschneider.com.
Features
Simulation methods
- Molecular dynamics (MD) in NVT, NPT, NVE ensembles
- Metropolis Monte Carlo (MC) in NVT and NPT ensembles JACS2004,JPhysChemB2006
- Replica exchange with MD and MC
Force fields
- OPLS-AA Jorgensen1996,Kaminski2001
Solvation models
- explicit solvent (water: TIP4P Jorgensen1985, SPC Berendsen1981)
- Generalized Born implicit solvent (GB/SA)
- Generalized Born implicit membrane (GB/IM) Proteins2007,BJ2007,Proteins2005
Enhanced productivity
A number of features make it easy to use Hippo so that one can spend more time on working on problems and less time on setting up structures or dealing with system crashes:
- seamless restarts
- intelligent pdb structure loader: reads most pdbs, can complete missing atoms, and builds the topology
Analysis
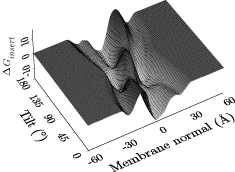
A growing number of analysis tools are built into Hippo, for instance
- adiabatic translation+rotation energy scan
- determines the Generalized Born energy of a peptide in a membrane MMB2008,BJ2006; this allows to decide if a (typically helical) peptide inserts into the membrane and at which depth and angle or if it prefers a surface-bound or even a fully solvated state
- RMSD
- calculates the root mean square deviation of trajectory frames to a reference structure
- helicity
- degree of helicity of segments along trajectory
- Z - tilt - kink graph
- Calculates center of mass, tilt angle, and kink angle of a peptide in a membrane as a function of simulation time. The membrane is in the xy plane, with z = 0 the membrane center. Kink angle is with respect to the membrane normal. Cordes2002
- cluster
- Performs a cluster analysis using the pairwise method by Daura et al.Daura1999
- fit to phase
- recenters trajectory on the centre of mass of a phase (such as a lipid membrane)
- fit solute to previous frames
- Generate a PDB movie by RMSD fitting the solute of each frame to the previous frame.
The Hippo output format is a binary xyz-movie (see the definition of the xyz format); thus many other analysis tools can also be used.
References
<biblio>
- MMB2008 pmid=18428040
- Proteins2007 pmid=17600830
- BJ2007 pmid=17218457
- BJ2006 pmid=16339877
- JPhysChemB2006 pmid=16913813
- Proteins2005 pmid=15723347
- JACS2004 pmid=14871118
- Kaminski2001 George A. Kaminski, Richard A. Friesner, Julian Tirado-Rives, and William L. Jorgensen. Evaluation and reparametrization of the OPLS-AA force field for proteins via comparison with accurate quantum chemical calculations on peptides. J. Phys. Chem. B, 105(28):6474–6487, 2001. 10.1021/jp003919d.
- Jorgensen1996 W. L. Jorgensen, D. S. Maxwell, and J. Tirado-Rives. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc., 118(45):11225–11236, 1996. 10.1021/ja9621760.
- Jorgensen1985 W. L. Jorgensen and J. D. Madura. Temperature and size dependence for Monte-Carlo simulations of TIP4P water. Mol. Phys., 56(6):1381–1392, December 1985.
- Berendsen1981 H. J. C. Berendsen, J. P. M. Postma, W. F. van Gunsteren, and J. Hermans. Interaction models for water in relation to protein hydration. In B. Pullman, editor, Intermolecular Forces, page 331. D. Reidel Publishing Company, Dordrecht, Holland, 1981.
- Cordes2002 pmid=12417206
- Daura1999 X Daura, K Gademann, B Jaun, D Seebach, WF van Gunsteren, and AE Mark. Peptide folding: When simulation meets experiment. Angewandte Chemie-International Edition, 38 (1-2):236–240, 1999. 10.1002/(SICI)1521-3773(19990115)38:1/2<236::AID-ANIE236>3.0.CO;2-M.
</biblio>